Imagine a world where plants hold the key to fighting off dangerous bacterial biofilms that wreak havoc in healthcare and industrial sectors. It may sound like science fiction, but researchers at UC Riverside have unearthed a remarkable discovery that could revolutionize how we combat these microbial menaces.
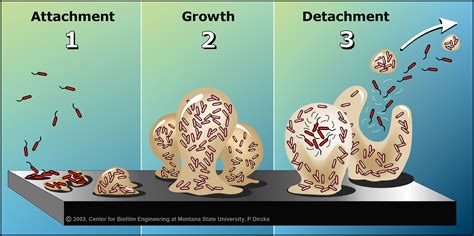
“In simple terms, biofilms are communities of microorganisms, like bacteria or fungi, that stick together and form a protective layer on surfaces,”
explains Katayoon Dehesh, a distinguished professor of molecular biochemistry at UCR. These slimy coatings you’ve encountered on river rocks or dental plaque are not just harmless decorations; they can pose serious threats when they take hold in medical devices or industrial equipment.
Published in the prestigious journal Nature Communications, the groundbreaking study sheds light on an unexpected hero: a metabolite known as MEcPP. This molecule is not only crucial for plant survival but also holds the power to thwart biofilm formation by bacteria such as E. coli. When plants face stress and accumulate MEcPP due to oxygen exposure from damage, it triggers protective responses within the plant – an innate defense mechanism against harm.
The research team’s discovery that MEcPP disrupts biofilm development by impeding bacterial attachment to surfaces opens up new avenues for combating infections and preventing corrosion in various industries. By targeting the initial stages of biofilm formation, this molecule shows promise in improving outcomes across sectors reliant on maintaining clean surfaces.
“Biofilms are like fortresses for bacteria,”
remarks Jingzhe Guo, UCR project scientist and lead author of the study. These resilient structures provide microbes with protection against antibiotics and cleaning agents, making infections harder to treat in medical settings while causing clogs and contamination in industrial pipelines and food processing equipment.
Through meticulous genetic screenings spanning thousands of bacterial mutants, the research team pinpointed a critical gene called fimE that serves as an “off switch” for fimbriae production – hair-like structures bacteria use to anchor themselves during biofilm initiation. The presence of MEcPP enhances fimE activity, reducing fimbriae production and hindering biofilm formation effectively disarming bacteria’s ability to construct their protective strongholds.
The implications of this discovery extend far beyond laboratories; they offer hope for more sustainable strategies in managing biofilms across diverse industries. Traditional approaches often rely on harsh chemicals or costly treatments with limited efficacy against adaptive bacteria over time. Harnessing nature’s own defense mechanisms through plant-derived molecules presents a promising alternative with significant environmental and economic benefits.
As Jingzhe Guo aptly summarizes,
“Our discovery could inspire biofilm prevention strategies across a wide range of industries.”
From enhancing water systems’ cleanliness to developing superior dental care products, the applications are boundless. This fusion of plant biology with microbiology exemplifies the unforeseen connections that spark innovation while offering a glimpse into how nature’s solutions can tackle human health challenges posed by bacterial threats.









Leave feedback about this